The correct reaction equation is:
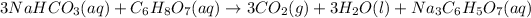
Answer:
b) 1 mole of water is produced for every mole of carbon dioxide produced.
Explanation: CONVERT EVERYTHING TO MOLES OR VOLUME, THEN COMPARE IT WITH THE COMPOUND'S STOICHIOMETRY IN CHEMICAL EQUATION.
a) 22.4 L of
 gas is produced only when
gas is produced only when
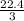 L of
L of
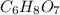 is reacted with 22.4 L of
is reacted with 22.4 L of
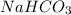 . So it is wrong.
. So it is wrong.
b) Since in the chemical equation the stoichiometric coefficient of
 and
and
 are same so the number of moles or volume of each of them will be same whatever the amount of reactants taken. Therefore it is correct option.
are same so the number of moles or volume of each of them will be same whatever the amount of reactants taken. Therefore it is correct option.
c)
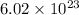 molecules is equal 1 mole of
molecules is equal 1 mole of
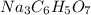 if produced then 3 moles of
if produced then 3 moles of
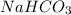 is required, which is not given in the option. So it is wrong.
is required, which is not given in the option. So it is wrong.
d) 54 g of water or 3 moles of
 (Molecular Weight of water is 18 g) is produced when 3 moles of
(Molecular Weight of water is 18 g) is produced when 3 moles of
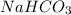 is used but in this option only one mole of
is used but in this option only one mole of
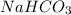 is given. So it is wrong.
is given. So it is wrong.