Answer:
Approximately 0.0100 L. That's equivalent to 10.0 mL.
Step-by-step explanation:
Look up the relative atomic mass data from a modern periodic table:
- H: 1.008;
- S: 32.06;
- O: 15.999.
Calculate the molar mass of
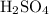 (sulfuric acid):
(sulfuric acid):
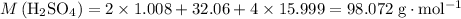 .
.
The standard unit of mass is gram. To make calculations easier, convert the mass of
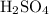 to that unit:
to that unit:
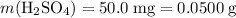 .
.
Find the number of moles of
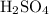 in this solution. Both the mass and the molar mass are in their respective standard unit. As a result, the value from this calculation will also be in the appropriate standard unit.
in this solution. Both the mass and the molar mass are in their respective standard unit. As a result, the value from this calculation will also be in the appropriate standard unit.
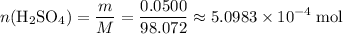 .
.
Calculate the volume of this solution. Note that for concentration,
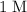 is the same as
is the same as
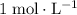 (moles per liter, not per milliliters)
(moles per liter, not per milliliters)
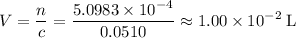 .
.
That's the same as
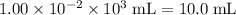 .
.