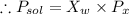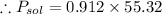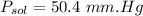Answer:
Step-by-step explanation:
According to given:
- molecular mass of glycerin,
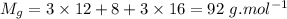
- molecular mass of water,
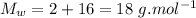
- ∵Density of water is
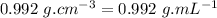
- ∴mass of water in 316 mL,
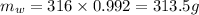
- mass of glycerin,
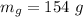
- pressure of mixture,
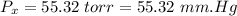
- temperature of mixture,
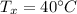
Upon the formation of solution the vapour pressure will be reduced since we have one component of solution as non-volatile.
moles of water in the given quantity:
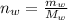
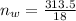
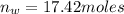
moles of glycerin in the given quantity:
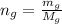
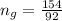
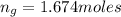
Now the mole fraction of water:
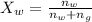
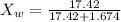
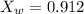
Since glycerin is non-volatile in nature so the vapor pressure of the resulting solution will be due to water only.