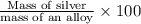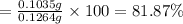Answer:
The percent of silver in the alloy is 81.87%.
Step-by-step explanation:
Mass of the alloy = 0.1264 g
Mass of silver chloride precipitate = 0.1375 g
Moles of silver chloride precipitate =
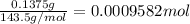
In 1 mole of silver chloride there are 1 mole of silver, then in 0.0009582 moles of silver chloride will ahve:
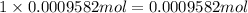 of silver
of silver
Mass of 0.0009582 moles of silver = 0.0009582 × 108 g/mol=0.1035 g
The percent of silver in the alloy: