Answer:
6.36 105 J
Step-by-step explanation:
In calorimetry all the energy given to a system is converted to heat and the equation for heat is
Q = m
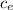 ΔT
ΔT
The temperature can be in degrees Celsius or Kelvin since the interval between them is the same, substitute and calculate
Q = 1.9 4186 (373-293)
Q = 6.36 105 J
This heat equals the energy supplied