Answer:
The final pressure of the gas when its temperature returns to its initial value
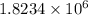 Pa.
Pa.
Explanation:
Given : An ideal gas is confined within a closed cylinder at a pressure of
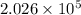 Pa by a piston. The piston moves until the volume of the gas is reduced to one-ninth of the initial volume.
Pa by a piston. The piston moves until the volume of the gas is reduced to one-ninth of the initial volume.
To find : What is the final pressure of the gas when its temperature returns to its initial value?
Solution :
Since the temperature is constant .
The relation between P and V is given by,
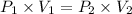
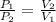 ....(1)
....(1)
The piston moves until the volume of the gas is reduced to one-ninth of the initial volume i.e.
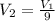
or
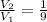
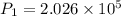
Substitute in equation (1),
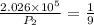
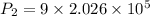
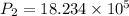
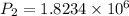
The final pressure of the gas when its temperature returns to its initial value
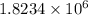 Pa.
Pa.