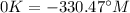Answer:
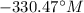
Step-by-step explanation:
Let's begin by explaining that the relation between the Celsius scale and Kelvin scale is:
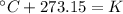
This means the absolute zero point of the Kelvin scale is
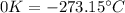
Keeping this in mind, we have the freezing point and boling point of Methane as:
Freezing point:
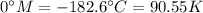
Boiling point:
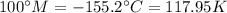
According to this, there is a linear relation between the methane scale (
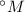 ) and the Kelvin scale in the form:
) and the Kelvin scale in the form:
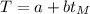 (1)
(1)
Where:
 is the temperature in Kelvin
is the temperature in Kelvin
 is the temperature in degrees Methane
is the temperature in degrees Methane
Firstly, we need to find the value of
 and
and
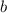 with the two given points (
with the two given points (
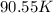 and
and
 ):
):
When
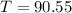 and
and
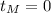 :
:
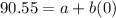
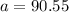 (2)
(2)
When
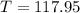 and
and
 :
:
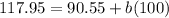
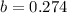 (3)
(3)
Now we have the linear equation:
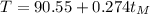 (4)
(4)
Isolating
 :
:
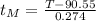 (5)
(5)
Evaluating for
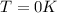 :
:
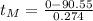 (6)
(6)
Finally:
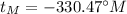
This means