Answer:
ΔH=-957.41 kJ
Step-by-step explanation:
The chemical equation is:
B₅H₉ (l) + O₂ (g) ⇒ B₂O₃ (s) + H₂O(l)
The law of conservation of matter states that since no atom can be created or destroyed in a chemical reaction, the number of atoms that are present in the reagents has to be equal to the number of atoms present in the products.
Then, you must balance the chemical equation. For that, you must first look at the subscripts next to each atom to find the number of atoms in the equation. If the same atom appears in more than one molecule, you must add its amounts
The coefficients located in front of each molecule indicate the amount of each molecule for the reaction. This coefficient can be modified to balance the equation, just as you should never alter the subscripts.
By multiplying the coefficient mentioned by the subscript, you get the amount of each element present in the reaction.
So in first place you balance B
2 B₅H₉ (l) + O₂ (g) ⇒ 5 B₂O₃ (s) + H₂O(l)
Then you balance H
2 B₅H₉ (l) + O₂ (g) ⇒ 5 B₂O₃ (s) + 9 H₂O(l)
Finally you balance O
2 B₅H₉ (l) + 12 O₂ (g) ⇒ 5 B₂O₃ (s) + 9 H₂O(l)
Then
Left side: 2*5=10 boron (B), 2*9=18 hydrogen. and 12*2=24 oxygen
Right side: 5*2=10 boron (B), 9*2=18 hydrogen. and 5*3 + 9*1=24 oxygen
Since you have the same amount of elements on each side of the equation, the equation is balanced.
You want to calculate the ∆H (heat of reaction) of the combustion reaction, that is, the heat that accompanies the entire reaction. For that you must make the total sum of all the heats of the products and of the reagents affected by their stoichiometric coefficient (quantity of molecules of each compound that participates in the reaction) and finally subtract them:
Enthalpy of combustion = ΔH = ∑Hproducts - ∑Hreactants
Knowing that:
- Heat of formation of B₅H₉ = 73.2 kJ/mol
- Heat of formation of water = -285.4 kJ/mol
- Heat of formation of B₂O₃ = -1,272 kJ/mol
For the formation of one mole of a pure element the heat of formation is 0, in this case we have as a pure compound the oxygen O₂.
Then:
ΔH= 9 mol*(-285.4 kJ/mol) + 5 mol* (-1,272 kJ/mol) - [2 mol* 73.2 kJ/mol + 12 mol* 0 kJ/mol]
ΔH= -9,075 kJ
If you observe the previous balanced reaction, you can see that 2 moles of B₅H₉ (l) are necessary. And the calculation of the heat of reaction previously carried out is based on this reaction. This ultimately means that the energy that would result in the reaction of 2 moles of B₅H₉ (l) is -9,075 kJ.
To determine the heat that is released when 0.211 mol of B₅H₉ (l) react with excess oxygen, a rule of three is applied as follows: if 2 moles of B₅H₉ (l) produces a heat ΔH of -9,075 kJ, when reacting 0.211 mol of B₅H l (l) how much heat ΔH is released?
ΔH=
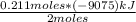
ΔH=-957.41 kJ