Answer:
The mass of Neptunium is 237.054 u.
Step-by-step explanation:
Given that,
Mass of Americium = 241.05682 u
Mass of alpha particle = 4.00260 u
The equation is,
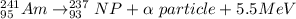
Let the mass of Neptunium is m.
Since the mass remain same.
We need to calculate the mass of Neptunium
Using formula of mass
Mass of Neptunium = Mass of Americium -Mass of alpha particle
Put the value into the formula
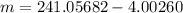
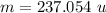
Hence, The mass of Neptunium is 237.054 u.