Answer: The number of nitrogen molecules in the container are

Step-by-step explanation:
To calculate the moles of gas, we use the equation given by ideal gas which follows:

where,
P = pressure of the gas = 780 mmHg
V = Volume of the gas = 400.0 mL = 0.4 L (Conversion factor: 1 L = 1000 mL)
T = Temperature of the gas =
![135^oC=[135+273]K=408K](https://img.qammunity.org/2020/formulas/chemistry/high-school/y4gyc2v3z3m2h7joucnlt790s3prdli92g.png)
R = Gas constant =
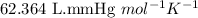
n = number of moles of nitrogen gas = ?
Putting values in above equation, we get:
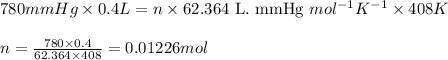
According to mole concept:
1 mole of a compound contains
 number of molecules
number of molecules
So, 0.01226 moles of nitrogen gas will contain =
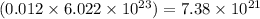 number of molecules
number of molecules
Hence, the number of nitrogen molecules in the container are
