Answer:
(a)The molar mass of the gene fragment is 18220.071g/mol = 18.22 kg/mol
(b)The freezing point for the aqueous solution is
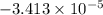 C
C
Step-by-step explanation:
The osmotic pressure (π) is given by the following equation:
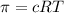
 = Concentration of solution
= Concentration of solution
R = universal gas constant = 62.364
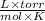
T = temperature
Weight of solute = w = 10.0 mg
Let the molecular weight of the solute be m g/mol.
Concentration =
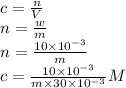
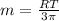
m = 18220.071g/mol
Therefore, the molar mass of the gene fragment is 18220.071g/mol = 18.22 kg/mol
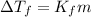
m is the molality of the solution.
m =
 mol/kg
mol/kg
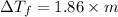
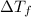 =
=
 C
C
The freezing point for the aqueous solution is
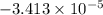 C
C