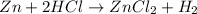Answer:
1. a chemical reaction in which one substance breaks up into two or more new substances: decomposition reaction
2. a reaction in which two or more substances combine to form a new substance: synthesis reaction
3. the reaction of an acid with a base to form a salt and water: neutralization reaction.
4. chemical compound formed when the negative ions from an acid combine with the positive ions of a base: salt
5. two ionic compounds reacting in solution to form two new compounds, one of which is insoluble: double displacement reaction.
6. a reaction in which an active metal displaces a less active metal or hydrogen from a compound solution (or a nonmetal replaces a nonmetal from a compound in solution): Single replacement reaction
Step-by-step explanation:
1. Decomposition is a type of chemical reaction in which one reactant gives two or more than two products.
Example:
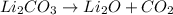
2. Synthesis reaction is a chemical reaction in which two reactants are combining to form one product.
Example:
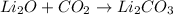
3 and 4. Neutralization is a chemical reaction in which an acid and a base reacts to form salt and water. Salt is formed when cations or positive ions of base combine with anions or negative ions of acid.
Here
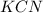 is the salt formed by combination of
is the salt formed by combination of
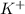 from base and
from base and
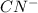 from acid.
from acid.
Example:
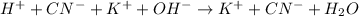
5. A double displacement reaction is one in which exchange of ions take place. The salts which are soluble in water are designated by symbol (aq) and those which are insoluble in water and remain in solid form are represented by (s) after their chemical formulas.
Example:
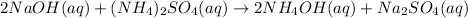
6. Single replacement reaction is a chemical reaction in which more reactive element displaces the less reactive element from its salt solution.
Example: