Answer: 181 kJ
Step-by-step explanation:
The balanced chemical reaction is;
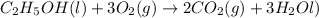
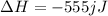
To calculate the moles, we use the equation:
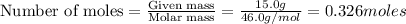
According to stoichiometry:
1 mole of
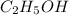 on complete combustion give= 555 kJ
on complete combustion give= 555 kJ
Thus 0.326 moles of
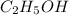 on complete combustion give=
on complete combustion give=
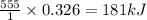
Thus the enthalpy change for combustion of 15.0 g of ethanol is 181 kJ