Answer:
1,812 wt%
Step-by-step explanation:
The reactions for this titration are:
2Ce⁴⁺ + 3I⁻ → 2Ce³⁺ + I₃⁻
I₃⁻ + 2S₂O₃⁻ → 3I⁻ + S₄O₆²⁻
The moles in the end point of S₂O₃⁻ are:
0,01302L×0,03428M Na₂S₂O₃ = 4,463x10⁻⁴ moles of S₂O₃⁻. As 2 moles of S₂O₃⁻ react with 1 mole of I₃⁻, the moles of I₃⁻ are:
4,463x10⁻⁴ moles of S₂O₃⁻×
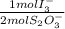 = 2,2315x10⁻⁴ moles of I₃⁻
= 2,2315x10⁻⁴ moles of I₃⁻
As 2 moles of Ce⁴⁺ produce 1 mole of I₃⁻, the moles of Ce⁴⁺ are:
2,2315x10⁻⁴ moles of I₃⁻×
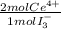 = 4,463x10⁻⁴ moles of Ce(IV). These moles are:
= 4,463x10⁻⁴ moles of Ce(IV). These moles are:
4,463x10⁻⁴ moles of Ce(IV)×
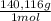 = 0,0625 g of Ce(IV)
= 0,0625 g of Ce(IV)
As the sample has a 3,452g, the weight percent is:
0,0625g of Ce(IV) / 3,452g × 100 = 1,812 wt%
I hope it helps!