Answer:
R=0·083 J/mol·K
Step-by-step explanation:
Ideal gas equation is
P×V = n×R×T
where,
P is the pressure of the gas
V is the volume of the gas
n is the number of moles of the gas
R is ideal gas constant
T is the temperature of the gas in Kelvin
In case of given problem
Temperature of the gas = 273+2·00=275·00K
P=259,392·00×
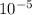 atm
atm
(259,392·00×
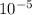 )×8·8 = 1×R×275·00
)×8·8 = 1×R×275·00
∴R=0·0830 J/mol·K
But according to the rules of significant figures the value of R must be with least precision of all the values of the other parameters from which the value of R is calculated
Here the least precision is in the volume as it contains only 2 significant digits
∴ Value of R must contain 2 significant digits
∴ R=0·083 J/mol·K