To solve this problem, it is necessary to apply the ideal Gas equations, as well as the calculation equations of the weight difference, which under the comparison of two values.
By definition we know that the ideal gas equation is given by the equation,
PV = nRT
Where,
P = Pressure
V = Volume
R = Gas ideal constant
T = Temperature
n = number of moles
Our values are given by
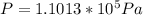
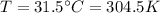
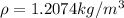
PART A) Using this previous equation we can find the number of moles per Volume, that is
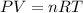
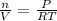
Replacing with our values
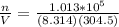
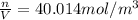
PART B ) We can calculate the number of moles of 1m^3 through Avogadro number, then
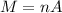
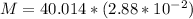
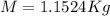
Therefore in
 there are 1.1524Kg of Gas.
there are 1.1524Kg of Gas.
PART C ) Density can be defined as the proportion of mass in a specific quantity of Volume, then
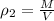
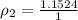
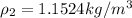
The difference of percentage then is
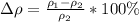
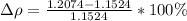
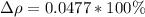
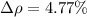
YES, because as the percentage is less than 10%, the calculated value agrees with the stated value.