Answer:
a. A reaction in which the entropy of the system increases can be spontaneous only if it is endothermic.
Step-by-step explanation:
The change in free energy (ΔG) that is, the energy available to do work, of a system for a constant-temperature process is:
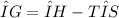
- When ΔG < 0 the reaction is spontaneous in the forward direction.
- When ΔG > 0 the reaction is nonspontaneous. The reaction is
spontaneous in the opposite direction.
- When ΔG = 0 the system is at equilibrium.
If both ΔH and ΔS are positive, then ΔG will be negative only when the TΔS term is greater in magnitude than ΔH. This condition is met when T is large.