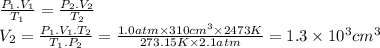Answer:
1.3 × 10³ cm³
Step-by-step explanation:
The gas occupies a volume of V₁ = 310 cm³ under standard temperature and pressure (STP), that is, T₁ = 273.15 K and P₁ = 1.0 atm. In order to find the volume V₂ under different conditions we can use the combined gas law formula.