Answer:
Limiting reactant is NaOH
Step-by-step explanation:
In the reaction:
2S(s) + 3O₂(g) + 4NaOH(aq) → 2Na₂SO₄(aq) + 2H₂O(l)
4.0 g of sulfur, 6.0 g of oxygen and 8.0 g of sodium hydroxide are:
4,0g S×
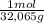 = 0,125 moles S
= 0,125 moles S
6,0g O₂×
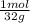 = 0,188 moles O₂
= 0,188 moles O₂
8,0g NaOH×
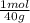 = 0,2 moles NaOH
= 0,2 moles NaOH
For a complete reaction of 0,2 moles of NaOH you will need:
0,2 mol NaOH×
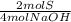 = 0,1 moles of Sulfur
= 0,1 moles of Sulfur
0,2 mol NaOH×
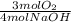 = 0,15 moles of Oxygen
= 0,15 moles of Oxygen
As you have 0,125 moles of S and 0,188 moles of O₂ that are more than moles you need for a complete reaction of NaOH, limiting reactant is NaOH
I hope it helps!