Answer:
the temperature of the gas must have increased
Step-by-step explanation:
As we know that
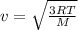
from this at room temperature the speed of air molecules is given as
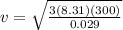
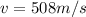
now if the speed of the container was initially at
v = 2000 m/s
now we know that this speed is more than the speed of air molecules at room temperature
so here the temperature of the gas must have increased