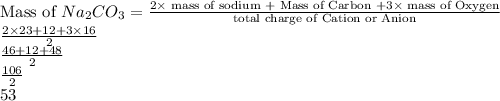It is easy to find the equivalent mass for this compound
.
divide the molar mass of the compound by the valence of the element/ion.
Since, Sodium shows +1 oxidation state always
and it's 2 times. so the total charge is +2
so just divide the molar mass by 2