Answer:
598.74 mL
Step-by-step explanation:
Using Ideal gas equation for same mole of gas as
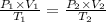
Given ,
V₁ = 530 mL
V₂ = ?
P₁ = 1.2 atm
P₂ = 1 atm
T₁ = 17 ºC
T₂ = 0 ºC
The conversion of T( °C) to T(K) is shown below:
T(K) = T( °C) + 273.15
So,
T₁ = (17 + 273.15) K = 290.15 K
T₂ = (0 + 273.15) K = 273.15 K
Using above equation as:
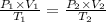
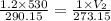
Solving for V₂ , we get:
V₂ = 598.74 mL