Answer:
(1) The absorbance of the aspirin in solutions will increase.
(2) [ASA]f = 3.79x10⁻⁴M
(3) [ASA]i = 3.79x10⁻³M
(4) m ASA = 0.171g
Step-by-step explanation:
The Beer's Law is expressed by:
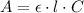 (1)
(1)
where A: is the absorbance of the species, ε: is the molar attenuation coefficient, l: is the pathlength and C: is the concentration of the species
(1) From equation (1), the relation between the absorbance of the species and its concentration is directly proportional, so if the aspirin concentration in solutions increases, the absorbance of the solutions will also increase.
(2) Starting in the given expression for the relationship between absorbance and concentration of ASA, we can calculate its concentration in the solution:
![A = 1061.5 \cdot [ASA]](https://img.qammunity.org/2020/formulas/chemistry/college/3t1k9wlomv0olhj9lhqjbswux2vwbrxph4.png)
![[ASA] = (A)/(1061.5) = 3.79 \cdot 10^(-4)M](https://img.qammunity.org/2020/formulas/chemistry/college/63g4s6k1tl4uttx8ydle8mk0jx2qjjltqb.png)
Therefore, the aspirin concentration in the solution is 3.79x10⁻⁴ M
(3) To calculate the stock solution concentration, we can use the next equation:
![V_(i) [ASA]_(i) = V_(f) [ASA]_(f)](https://img.qammunity.org/2020/formulas/chemistry/college/y1laehvu4humk3uo7ojl1dd8wx9m0vj0x8.png)
where Vi: is the stock solution volume=10mL, Vf: is the solution diluted volume=100mL, [ASA]i: is the aspirin concentration of the stock solution and [ASA]f: is the aspirin concentration of the diluted solution
![[ASA]_(i) = (V_(f) \cdot [ASA]_(f))/(V_(i)) = \frac {100mL \cdot 3.79\cdot 10^(-4) M}{10mL} = 3.79 \cdot 10^(-3) M](https://img.qammunity.org/2020/formulas/chemistry/college/2bl0x2ubd7eoj2895zmtznu1n611hjzs5v.png)
Hence, the concentration of the stock solution is 3.79x10⁻³M
(4) To determine the aspirin mass in the tablet, we need to use the following equation:
![m_(ASA) = \eta_(ASA) \cdot M_(ASA) = [ASA]_(i) \cdot V_(0) \cdot M_(ASA)](https://img.qammunity.org/2020/formulas/chemistry/college/sq7xi9ix7dbqc76eydndupwd7qphagn4zk.png)
where η: is the aspirin moles = [ASA]i V₀, M: is the molar mass of aspirin=180.158g/mol, V₀: is the volume of the volumetric flask=250mL and [ASA]i: is the aspirin concentration in the volumetric flask which is equal to the stock solution=3.79x10⁻³M
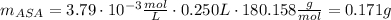
Then, the aspirin mass in the tablet is 0.171 g.
I hope it helps you!