Answer:
0,1966L
Step-by-step explanation:
The reaction of aluminium with HCl is:
Al(s) + 3HCl(aq) → AlCl₃ + ³/₂H₂(g)
Moles of aluminium that react are:
0,1453g×
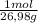 = 5,385x10⁻³ moles of Al
= 5,385x10⁻³ moles of Al
As 1 mole of Al produce ³/₂ moles of H₂(g), the moles of H₂ are:
5,385x10⁻³ moles of Al×
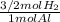 = 8,078x10⁻³ mol H₂(g)
= 8,078x10⁻³ mol H₂(g)
To find the volume H₂ occupies you need to use:
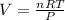
Where n are moles (8,078x10⁻³ mol H₂(g))
R is gas constant (0,082atmL/molK)
T is temperature (17,0°C+273,15 = 290,15K)
And P is pressure (29,25 inches of Hg/29,921= 0,9776 atm)
Replacing:
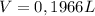
I hope it helps!