Step-by-step explanation:
A balanced equation is defined as the equation which contains the same number of atoms on both reactant and product side.
For example, when solid state of iron chemically reacts with chlorine gas then it results in the formation of a solid ferric chloride.
The chemical equation for this reaction is as follows.
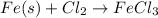
Number of atoms on reactant side are as follows.
Number of atoms on product side are as follows.
Hence, to balance this equation we will multiply Fe by 2 and
 by 2 on the reactant side. Also, we multiply
by 2 on the reactant side. Also, we multiply
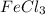 by 2 on product side.
by 2 on product side.
Therefore, the net balanced equation is as follows.
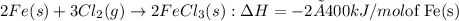
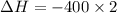 = -800 kJ
= -800 kJ