Step-by-step explanation:
The given data is as follows.
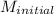 = 0.50 M,
= 0.50 M,
 = 3.00 ml
= 3.00 ml
 = 7.00 ml,
= 7.00 ml,
Therefore, calculate the total volume as follows.
Total volume =
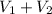
= 3.00 ml + 7.00 ml
= 10.0 ml
Now, calculate the no. of moles of acetic acid as follows.
No. of moles of acetic acid = molarity × volume in mL
= 3.0 × 0.50
= 1.5 mmoles
=
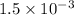 moles
moles
(As 1 mole = 0.001 mmol)
Therefore, calculate the concentration of acetic acid as follows.
Concentration of the acetic acid =
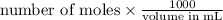
=
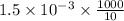
= 0.15 M
Thus, we can conclude that the concentration of acetic acid in buffer solution is 0.15 M.