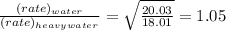Answer:
relative rate of diffusion is 1.05
Step-by-step explanation:
According to Graham's law of difussion:
Rate of diffusion is inversely proportional to the square root of molecular weight of a molecule.
For two given molecules:
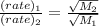
The given molecules are
Water = 18.01
Heavy water =20.03
Thus the relative rate of diffusion will be: