Answer:
The four quantum number for each electron will be:
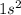
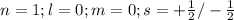
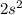
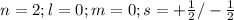
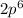
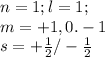
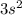
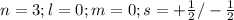
Step-by-step explanation:
As the element is neutral, the number of protons will be equal to number of electrons which will be the atomic number of the element.
Number of electrons =12
Atomic number = 12
Element : Magnesium
The principal shell is represented by "n"
i) For "s" subshell the value of l =0 (azimuthal quantum number) thus m (magnetic quantum number)= 0
The two electrons in s subshell will have either plus half or minus half spin quantum number
ii) for "p" subshell the value for l =1
thus m = 0 or +1 or -1
The two electrons in each orbital will have either plus half or minus half spin quantum number