Step-by-step explanation:
The given data is as follows.
T =
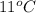 = (11 + 273) K = 284 K, V = 45.0 L
= (11 + 273) K = 284 K, V = 45.0 L
m = 35 g
As molar mass of chlorine pentafluoride is 130.445 g/mol. Hence, number of moles of chlorine pentafluoride are as follows.
No. of moles =

=
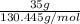
= 0.268 mol
Now, using the ideal gas equation we will find the pressure as follows.
PV = nRT
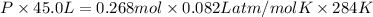
P = 0.139 atm
Thus, we can conclude that pressure of chlorine pentafluoride gas in the given reaction vessel after the reaction is 0.139 atm.