Answer:
The energy difference is 158.318 kJ/mol
Step-by-step explanation:
The energy can be calculated by the following formula.
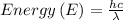
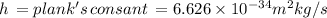
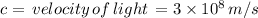
Let's calculate the energy at maximum:
Maximum energy =
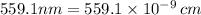
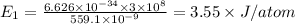
Let's calculate the energy at minimum:
Minimum energy =
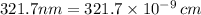
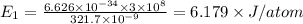
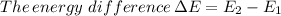
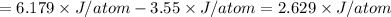
The number molecules present in one mole is
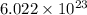
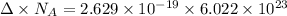
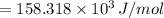
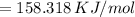
Therefore, the energy difference is 158.318 kJ/mol.