To develop this problem it is necessary to apply the concepts developed by Clausius - Claperyron.
This duet found the relationship between temperature and pressure expressed as,
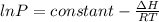
For the two states that we have then we could define the pressure and temperature in each of them as
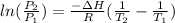
Where,
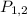 = Pressure at state 1 and 2
= Pressure at state 1 and 2
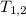 = Temperature at state 1 and 2
= Temperature at state 1 and 2
 = Enthalpy of Vaporization of a substance
= Enthalpy of Vaporization of a substance
R = Gas constant (8.134J/mol.K)
Our values are given by,
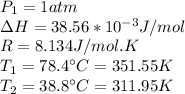
Therefore replacing we have that,
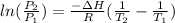
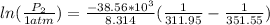
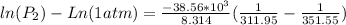
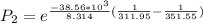
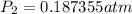
Therefore the pressure of the Ethanol at 38.8°C is 0.187355atm