Answer:

Step-by-step explanation:
The first step is to draw the ionization reaction of
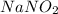 , so:
, so:

The molar ratio between
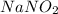 and
and
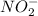 is 1:1
is 1:1
The next step is the calculation of the concentration. We have to remember that the formula of ppm is:

In this case we will have a mass of 150 mg and a volume of 1 L so:
