Answer:
The number of oxygen molecules in the left container greater than the number of hydrogen molecules in the right container.
Step-by-step explanation:
Given:
Molar mass of oxygen,
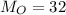
Molar mass of hydrogen,
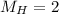
We know ideal gas law as:

where:
P = pressure of the gas
V = volume of the gas
n= no. of moles of the gas molecules
R = universal gs constant
T = temperature of the gas
∵
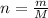
where:
m = mass of gas in grams
M = molecular mass of the gas
∴Eq. (1) can be written as:
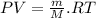
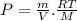
as:
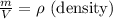
So,
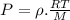
Now, according to given we have T,P,R same for both the gases.
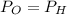
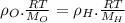
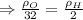
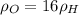
∴The molecules of oxygen are more densely packed than the molecules of hydrogen in the same volume at the same temperature and pressure. So, the number of oxygen molecules in the left container greater than the number of hydrogen molecules in the right container.