Answer:
The electron took two jumps to return to its ground state.
Step-by-step explanation:
If the electron is in nth state then it first goes to (n-1)th to 2nd state and emits one photon of this energy gap. Then, it goes to ground state and emits another photon of different energy. It can emit
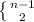 types of different photons. Fourth option is incorrect because in that case it emits one photon and absorbs one photon to go back to that state and also both photons are of same energy.
types of different photons. Fourth option is incorrect because in that case it emits one photon and absorbs one photon to go back to that state and also both photons are of same energy.