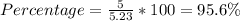Answer:
95.6 %
Step-by-step explanation:
For this question, we will have 2 reactions, the formation of the grignard reagent and the formation of the alcohol. The first step then is the calculation of the maximum amount of the grignard reagent. For this, we have to convert the grams to moles and check the smallest value. To do this we have to take into account the following conversion ratios:
Molar mass of Mg = 24 g/mol
Molar mass of phenylmagnesium bromide (
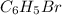 )= 157 g/mol
)= 157 g/mol
Density of bromobenzene= 1.5 g/mL
Molar ratio between Mg and
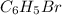 = 1:1
= 1:1
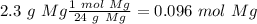
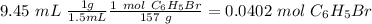
The smallest value is the mol of bromobenzene therefore 0.0402 mol of phenylmagnesium bromide would be produced.
The next step is repite the same steps for the reaction of formation of the alcohol. Therefore we have to find the moles of methyl benzoate, so:
Molar mass of methyl benzoate: 136.14 g/mol
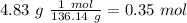
The we have to divide by the coefficient of each reactive in the balance reaction. So:
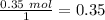
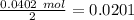
Therefore the limiting reagent would be the phenylmagnesium bromide. Now, the molar ratio between the phenylmagnesium bromide and triphenyl carbinol is 2:1, so the amount of alcohol produced is 0.0201 mol triphenyl carbinol. The next step is the conversion from mol to grams of triphenyl carbinol:
Molar mass of triphenyl carbinol= 260.33 g/mol
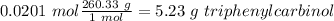
Finally, we have to divide the obtanied solid by the calculated one: