In 370 K to 375 K temperature intervals of 5 K, would be the greatest increase in the entropy of the sample.
Option: C
Step-by-step explanation:
Because the largest difference in molar entropy occurs when a condensed phase (solid/liquid) transforms to the gas phase. Then change in entropy is equal to heat transfer divided by temperature:
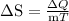 .
.
According to given ice sample at 260 K, when this solid sample start converting into liquid sample it will gain positive temperature and steam will take place near 373 K (273 K ice temperature +
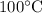 temperature of boiling water). Therefore it’s very obvious that greatest increase in entropy will occur during 370 K – 375 K.
temperature of boiling water). Therefore it’s very obvious that greatest increase in entropy will occur during 370 K – 375 K.