Answer:
For Kp = 1,4;
![P_{[A] = 0,22](https://img.qammunity.org/2020/formulas/chemistry/college/noz1fiaswpft1o04jyp74313bgc5h04kha.png) ,
,
![P_{[B] = 0,56atm](https://img.qammunity.org/2020/formulas/chemistry/college/1gsz0pbaa5tsxmaozduzj3ndcn827vq4jn.png)
For Kp = 2,0x10⁻⁴;
![P_{[A] = 0,495](https://img.qammunity.org/2020/formulas/chemistry/college/ot72peol9wpwyepzfdgrzidlelltyodoav.png) ,
,
![P_{[B] = 0,01atm](https://img.qammunity.org/2020/formulas/chemistry/college/d0lperep8yzccdw4m6lykesh6o2e6lwkmd.png)
For Kp = 2,0x10⁵;
![P_{[A] = 5x10^(-6)](https://img.qammunity.org/2020/formulas/chemistry/college/gdej615w9n6ocqcskd9uqdtvbq0816azlx.png) ,
,
![P_{[B] = 0,99999atm](https://img.qammunity.org/2020/formulas/chemistry/college/y6uv2wzh5v2mgtzr5508hvg1v59i99afn4.png)
Step-by-step explanation:
For the reaction:
A(g) ⇄ 2B(g)
kp is:
![kp = (P_([B])^2)/(P_([A]))](https://img.qammunity.org/2020/formulas/chemistry/college/mlp7ux4sgqdwrn3mz338kjvu08uvnd907m.png)
If initial pressure of B is 1,0atm and initial pressure of A is 0,0atm the equilibrium pressures are:
![P_{[A] = 0,0atm + X](https://img.qammunity.org/2020/formulas/chemistry/college/q5u1l36sxdp2a69bki5kyo2l1anoruwlx4.png)
![P_{[B] = 1,0atm - 2X](https://img.qammunity.org/2020/formulas/chemistry/college/fpx5s7k3ja9txewfzclqkn5b9rkdqeylfz.png)
Replacing for Kp= 1,4:
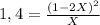
1,4X = 4X² - 4X + 1
0 = 4X² - 5,4X + 1
Solving for X:
X = 0,22 -Right answer-
X = 1,13
Replacing:
![P_{[A] = 0,22](https://img.qammunity.org/2020/formulas/chemistry/college/noz1fiaswpft1o04jyp74313bgc5h04kha.png)
![P_{[B] = 1,0atm - 0,44atm = 0,56atm](https://img.qammunity.org/2020/formulas/chemistry/college/ixs0uph7vcygr22hyyt93rokhdpxxx7qot.png)
For Kp= 2,0x10⁻⁴:
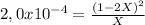
2,0x10^{-4}X = 4X² - 4X + 1
0 = 4X² - 4,0002X + 1
Solving for X:
X = 0,495atm
Replacing:
![P_{[A] = 0,495atm](https://img.qammunity.org/2020/formulas/chemistry/college/z59d6wnbb39i08pw8humzozj27dqunzmic.png)
![P_{[B] = 1,0atm - 0,99atm = 0,01atm](https://img.qammunity.org/2020/formulas/chemistry/college/vyqthcknp5hgk4k3d3310djleudunpbngq.png)
For Kp= 2,0x10⁵:
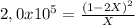
2,0x10^5X = 4X² - 4X + 1
0 = 4X² - 2,00004x10^5X + 1
Solving for X:
X = 5x10⁻⁶ -Right answer-
Replacing:
![P_{[A] = 5x10^(-6)](https://img.qammunity.org/2020/formulas/chemistry/college/gdej615w9n6ocqcskd9uqdtvbq0816azlx.png)
![P_{[B] = 1,0atm - 0,00001atm = 0,99999atm](https://img.qammunity.org/2020/formulas/chemistry/college/794n8w6c1jj9qbc7dfm5f82gi73ncfwuiz.png)
I hope it helps!