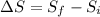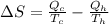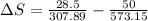To solve this problem it is necessary to take into account the Efficiency values in the energy cycles.
Efficiency can be defined as,
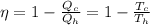
Where,
Q = Heat Exchange
T = Temperature
The efficiency for this system is 0.43 then,
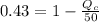
Re-arrange to find
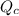
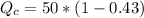
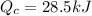
And the temperature would be given as,
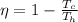
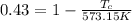
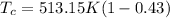
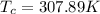
The entropy would be given as