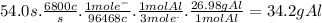Answer:
34.2 g
Step-by-step explanation:
In the Hall-Heroult process, Al³⁺ (from Al₂O₃) is reduced to Al. The reduction half-reaction is:
Al³⁺ + 3 e⁻ ⇒ Al
We can establish the following relations:
- 1 A = 1 c/s
- 1 mole of e⁻ has a charge of 96468 c (Faraday's constant)
- 1 mol of Al is produced when 3 moles of e⁻ circulate
- The molar mass of Al is 26.98 g/mol.
Suppose a current of 6800 A is passed through a Hall-Heroult cell for 54.0 seconds. The mass of Al produced is: