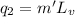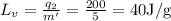40 J/g is the heat of vaporization of the liquid.
Answer: Option D
Step-by-step explanation:
Given that mass of liquid sample: m = 10 g
And, Specific heat of the liquid: S = 2 J/g K
Also, the increase in the temperature of the liquid,
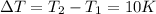
Therefore, the total amount of heat energy required is given by:
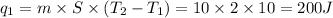
According to the given data in the question,
Total heat energy supplied, q = 400 J
Rest of heat would be
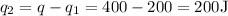
Now, 200 J vaporizes the mass, half of the liquid from full portion boiled away. So,
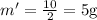
Latent heat of vaporization of the liquid is
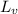 . It can be calculated as below,
. It can be calculated as below,