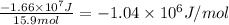Answer:
-1.04 × 10⁶ J/mol
Step-by-step explanation:
According to the law of conservation of the energy, the sum of the heat released by the combustion and the heat absorbed by the water is zero.
Qcomb + Qw = 0
Qcomb = - Qw [1]
The heat absorbed by the water can be calculated using the following expression.
Qw = cw . mw . ΔT
where,
cw is the specific heat capacity of the water
mw is the mass of the water
ΔT is the change in the temperature
Qw = (4.184 J/g.°C) × 98.6 × 10³ g × (80.7°C - 40.5°C) = 1.66 × 10⁷ J
From [1]
Qcomb = - 1.66 × 10⁷ J
The heat of combustion for this fuel is: