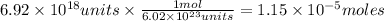Answer:
The number of moles of AgNO₃ is 1.15×10⁻⁵ moles.
Step-by-step explanation:
Accordig to the Avogadro's Law, equal volumes of different gaseous substances, measured under the same pressure and temperature conditions, contain the same number of particles.
Therefore, we can obtain that one mole of a certain substance contains 6.02×10²³ units.
The number of moles of AgNO₃ is given by: