Answer:
The reaction
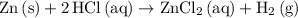 is spontaneous (under standard conditions) because the cell potential is positive.
is spontaneous (under standard conditions) because the cell potential is positive.
Step-by-step explanation:
In this reaction:
- Metallic zinc
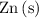 is oxidized to produce zinc ions
is oxidized to produce zinc ions
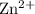 .
. - Hydrogen ions
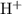 is reduced to produce hydrogen gas
is reduced to produce hydrogen gas
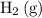 .
.
The anode is where the oxidation half-reaction takes place. In this case, the anode half-reaction is
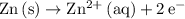 .
.
The cathode is where the reduction half-reaction takes place. In this case, the cathode half-reaction is
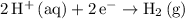 .
.
Look up the standard reduction potential for these two half-reactions:
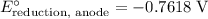 for the reaction
for the reaction
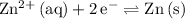 .
.
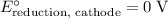 for the reaction
for the reaction
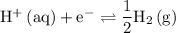 .
.
The standard cell potential is equal to the standard reduction potential at the cathode minus that at the anode:
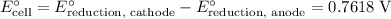 .
.
The electrochemical reaction in the cell is spontaneous if and only if the cell potential is positive. Therefore, the reaction
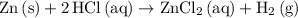 is spontaneous under standard conditions.
is spontaneous under standard conditions.