Answer:
The temperature of the water is 70°C
Step-by-step explanation:
Partial pressure of helium gas = 526 mmHg
Total pressure inside the jar= 760 mmHg
Suppose partial pressure of helium gas is
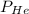 and partial pressure of water is
and partial pressure of water is
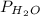
And total pressure is
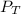
As we know
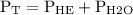
Hence
Putting the values
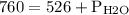
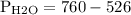
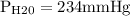
In bar,
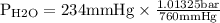
= 0.312 bar
Now according to the steam table
With the vapour pressure of water as 0.312 bar the temperature corresponds to 70°C