Answer : The correct option is, (B) two covalent bonds
Explanation :
Ionic bond : It is defined as the bond that is formed by complete transfer of electrons from one atom to another atom.
That means, the atom which looses the electron is known as electropositive atom and the atom which gains the electron is known as electronegative atom.
The ionic bond is usually formed between a metal and a non-metal.
Covalent bond : It is defined as the bond which is formed when sharing of electrons takes place between the atoms.
This is usually formed between two non-metals.
Metallic bond : It is defined as the bond that is formed from the electrostatic attraction between the free electrons and positively charged metal ions.
As per question, the two chemical bonds unite three atoms into a molecule, it is formed by the covalent bond.
For example :
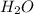 is formed by the combination of 2 hydrogen and 1 oxygen atom by the two covalent bonds because hydrogen and oxygen is a non-metal element.
is formed by the combination of 2 hydrogen and 1 oxygen atom by the two covalent bonds because hydrogen and oxygen is a non-metal element.
Hence, the correct option is, (B) two covalent bonds