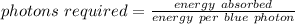So,
We are given:
mass of plate = 0.50 kg
specific heat capacity = 840 J/(kg °C)
wavelength of infrared = 18 * 10^-5 m
wavelength of blue light = 4.6 * 10^-7 m
ΔT = +2.0 °C
Additionally, we should already know:
q = mcΔT, where q is the energy absorbed (+) or released (-) by the system, m is the mass of the system, c is the specific heat capacity, and deltaT is the change in temperature.
E = hv, where E is the energy in J, h is Planck's constant, and v is the frequency of the light
c = wv, where c is the speed of light, w is the wavelength, and v is the frequency
We need to find the number of infrared photons and the number of blue photons required to result in the given temperature change.
Key idea: if we can find the energy if each photon, we can find the number of photons required to raise the temperature of the plate.
I will start with the infrared photons. We can do this with the assumed equations. We want to find E. We have w, and we should have h and c.
First, let's find v.
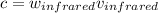
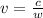

Next, let's find E.

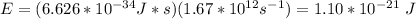
Now, let's find the amount of energy absorbed by the plate.
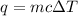

Now, we can find the number of infrared photons required.
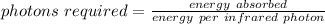
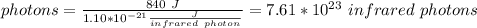
So the number of infrared photons required is 7.57 * 10^23 photons.
We can do a similar procedure for the blue light.
Find v.
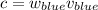
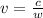
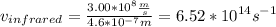
Find E.

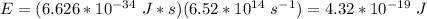
The energy absorbed by the plate is the same 840 J.
Now, find the number of blue photons required.