Answer : The amount of heat needed is, 9405 J
Explanation :
Formula used :
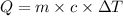
or,
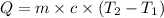
where,
Q = heat required = ?
m = mass of ice = 150 g
c = specific heat of water =
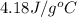
 = initial temperature =
= initial temperature =
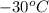
 = final temperature =
= final temperature =
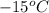
Now put all the given value in the above formula, we get:
![q=150g* 4.18J/g^oC* [(-15)-(-30)]^oC](https://img.qammunity.org/2020/formulas/chemistry/middle-school/evhz9t6u1x9vz307k4qau6aaqk8bmuzi8s.png)
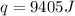
Therefore, the amount of heat needed is, 9405 J