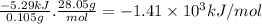Answer:
A. –1.41 × 10³ kJ/mol
Step-by-step explanation:
According to the law of conservation of energy, the sum of the heat released by the combustion and the heat absorbed by the calorimeter is zero.
Qcal + Qcomb = 0
Qcomb = -Qcal [1]
We can calculate the heat absorbed by the calorimeter using the following equation:
Qcal = Ccal × ΔT
where,
Ccal is the heat capacity
ΔT is the change in the temperature
From [1]
Qcomb = -Qcal = -Ccal × ΔT = -2.47 kJ/K × 2.14 K = -5.29 kJ
We know that the molar mass of ethylene is 28.05 g/mol. Then, the heat released per mole of ethylene is: