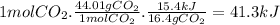Answer:
ΔHrxn = 41.3 kJ
Step-by-step explanation:
Let's consider the following equation.
CO₂(g) + H₂(g) ⇒ CO(g) + H₂O(g)
When 16.4 g of CO₂ react 15.4 kJ of energy are absorbed. In the balanced equation, there is 1 mole of CO₂, so we have to calculate the heat absorbed per mole of CO₂. Taking into account that the molar mass of CO₂ is 44.01 g/mol, the enthalpy of reaction (ΔHrxn) is: