Answer: [tex]CO_2(s)\rightarrow CO_2(g)[/tex]
Step-by-step explanation:
Entropy is the measure of randomness or disorder of a system. If a system moves from an ordered arrangement to a disordered arrangement, the entropy is said to decrease and vice versa.
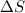 is positive when randomness increases and
is positive when randomness increases and
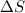 is negative when randomness decreases.
is negative when randomness decreases.
a)
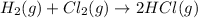
2 molesof gas are converting to 2 moles of another gas , thus
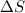 is zero.
is zero.
b)
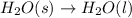
1 mole of solid is converting to 1 mole of liquid, the randomness increases and thus
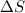 is positive.
is positive.
b)
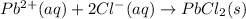
2 moles of ions are converting to 1 mole of solid, the randomness decreases and thus
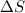 is negative
is negative
d)
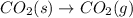
1 mole of solid is converting to 1 mole of gas, the randomness increases drastically and thus
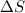 is highly positive.
is highly positive.